My Muskokan Mystery Mushroom
Here is a curious individual which escaped identification on the Cain Foray in Muskoka last weekend. Anyone out there have a theory? The experts said it was too difficult to ID because of a lack of info as it was not mature enough, and as I had not retrieved the base, it was unclear whether it was growing on the wood or debris.
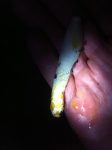
It was found growing horizontally out of the side of a hollow tree gall or burl about the size of a cantaloupe on the side of a sugar maple. The 6″ diameter tree had three such large growths, only one of which had an opening. I was unable to retrieve the base, but the firmness of its attachment suggested it was growing on the wood and not on an accumulation of plant matter, or an insect, inside the hollow. It is interesting to theorize on the mushroom’s relation to the gall.
- Was the fungus the cause of the growth
- Was it parasitizing the insect or bacteria that caused the growth?
- Attacking the tree in a vulnerable location?
- Growing on debris accumulated inside the gall?
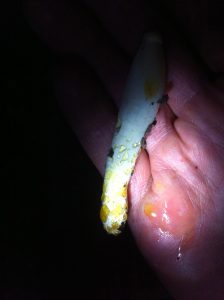
There was a drop of blood red exudate near the tip, and lots of yellow exudate around the bottom half of the stem. I don’t recall it having a distinct smell or not.
I found it late on the first the day, hence the dark pictures. The poor condition of the specimen on arrival at the ID table, and these crappy pictures taken with my iPhone and a flashlight, were not much help. My first indication that as a newbie, I had come ill-prepared for the foray.
Late Breaking Update Sept 22: Thanks to Jan Thornhill of Mycological Society of Toronto on the FaceBook Mushroom Identification Forum for solving the mystery and the correct answer above was # 3. See our discussion below:
- Inside a hollow growth on the side of a sugar maple. Drops of red and yellow exudate visible.
- Red and yellow exudate
- Lots of yellow liquid around the base
- Waiting for the identification which never came ;-(
Jan Thornhill I think it was a baby Lentils levis – it certainly yellowed as it dried on the table.
Mycognostic Thanks Jan. Looks like you got spell checked there. I’m checking out the Lentinus levis looking for “Baby Pictures”
Jan Thornhill Definitely not lentils! I’m on laptop and spellcheck is still on.
Mycognostic Well it definitely was tenacious and it sure meets a lot of Lentinus levis’ characteristics in terms of habit and location, but no mention of secretions.
But if it was basically still in primordial form (as it was still in the low light/high CO2 environment of the burl), it was in its pre-cap stage and sweating exudate which isn’t mentioned when describing the mature mushroom. That’s something I’ve seen in cultivated mushroom of various species.
http://www.messiah.edu/…/species…/Lentinus%20levis.htm
Lentinus levis
Scientific name: Lentinus levis (Berk. & M.A. Curtis) MurrillDerivation of name: Lentus means “pliable” and “tenacious” as in chewy. Levis means polished or smooth. Synonyms: Pleurotus levis (Berk. & M.A. Curtis) Singer; Panus strigosus Berk. & M.A. Curtis; Panuslevis Berk. & M.A. Curtis Common na…
messiah.edu
Mycognostic Thanks again Jan. I haven’t found any pics of primordial L. levis but that’s not surprising. I have attached a picture of a G. lucidum primordium secreting a fluid that is usually not mentioned in describing the mature fruit body.
Jan Thornhill Guttation/exudiate is hardly ever mentioned, even when it is diagnostic, i.e. for Inonotus glomeratus that bleeds pretty spectacular black tarry drops.